30 Which of the Following Is a Binary Molecular Compound
All elemental molecules are made of atoms of a single element. Which of the following compounds is a binary molecular compounda.

Chapter 9 Chemical Names And Formulas Ppt Video Online Download
Chemistry 26122019 2231 kingjaheem4109.

. Although there room no ion in this compounds lock are called in a comparable manner come binary ionic compounds. ACoH2PO42KClPCl3 bKIAl203Li2S CH20Si02NO dCoCl2CaCO3AlPO4. Sodium chloride and potassium hydroxide are certainly binary containing only TWO or THREE types of atoms but they are NOT molecular and consist of infinite arrays of positively and negatively charged ions the which are held together by electrostatic interaction.
Get the detailed answer. Magnesium has a 2- oxidation number and chlorine has a 1 oxidation number. Usually the least electronegative element is placed first CO2 BF3 etc.
Get solutions Get solutions Get solutions done loading Looking for the textbook. A binary compound is a compound composed of only two elements ie a binary compound is made from exactly two different elements. H2O is indeed a binary molecular compound.
These examples show how the rules are applied for the covalent. This contrasts with ionic compounds which are formed from a metal ion and a nonmetal ion. N the scope of its subject chemistry occupies an intermediate position.
Asulfur hexaflouride bwater cphosphorus trichloride dpotassium sulfide. Use the appropriate naming convention for ionic or molecular substances to assign a name to each compound. Select all that apply.
The nonmetal elements are. -Binary acids are named using the prefix hydro--The name of a binary acid contains the nonmetal root with the suffix -ic Which of the following statements correctly describe how to name a binary molecular compound. 0 followers 0 following Joined March 2017.
The elements that combine to form binary molecular compounds are both nonmetal atoms. Which of the following sets is comprised exclusively of ionic binary compounds. A binary molecular compound is a molecular compound that is composed of two elements.
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride. Which of the following compounds is a binary molecular compound. 1 Ionic binary compound.
Binary molecular compounds are compounds that consist of exactly two nonmetal elements. Binary acids are binary compounds whose chemical formula is eqH-X eq where eqH eq is hydrogen and eqX eq is any nonmetal element. Binary compound which is formed by the combination of a metal atom and non metal atom is called ionic binary compound eg KBr NaCl C a I 2 A l F 3 M g B r 2.
Compound name Molecular weight. Binary acids are capable of liberating their. Which of the following is a binary molecular compound A.
Which of the following is a binary ionic compound. Their composition structure properties behavior and the changes they undergo during a reaction with other substances. The solid is a molecular solid having NaCl structure.
Which of the following is a binary molecular compound. A binary compound is a compound that contains only two elements. A AgI B BeHCO3 C PCL5 D MgS.
A binary molecular compound is a compound that is form by 2 nonmetal elements. -The name of the first element remains unchanged except for the addition of a prefix where appropriate. Chemistry is the scientific discipline involved with elements and compounds composed of atoms molecules and ions.
Hydrogen H2 oxygen O2 and chlorine Cl2 molecules for example each contains two atoms. The reason that it may be slightly confusing to see that is because most people know it by its accepted common name of Water To sound much more. A T i C l 4 and C a F 2 b C l F 3 and V F 3 c S b C l 5 and A l F 3.
HClO2 chloric acid B. The number of valence electrons in silicon is ____ a8. This translates into placing the element.
B CNOF SIP SCl As Se Br Te L At and H. In naming a binary molecular compound the number of atoms of each element present in the molecule is indicated by ____. Which of the following is an example of a molecular element.
A b c d. In the following pairs of binary compounds determine which one is a molecular substance and which one is an ionic substance. On the other hand CO2 is composed of oxygen and carbon atoms.
FeO is a black solid insoluble in water and analogous to FeS. Organic and Biological Chemistry Loose-leaf Version 7th Edition Edit edition Solutions for Chapter 512 Problem 1QQ. Which element comes first in a binary molecular compound with both are from the same group in the name and formula.
Which of the following is a binary molecular compound. Examples include HF NO 2 and P 2 O 5Naming binary molecular compounds is really quite easy. The nomenclature that binary covalent compounds complies with these rules.
BeHCO3 AgI PCI5 MgS - 30000922 BeHCO3 AgI PCI5 MgS - 30000922 caitlynngronenthal caitlynngronenthal. Another form of oxygen ozone O3 has three atoms and sulfur S8 has eight atoms. 33 rows A B.
Which of the following is a binary molecular compound. They are located on the right side of the periodic table with the exception of H that is on the top left because it shares properties with the elements that are on that side of the periodic table. Binary molecular covalent compound are formed as the result of a reaction between two nonmetals.
BeF2 H3PO4 phosphoric acid Which of the following shows both the correct formula and correct name of an acid A. 1 Show answers Another question on Chemistry. Which of the following is a binary molecular compound.
There are two types of binary compounds. Binary Molecular Compound Names. As we can see C and O.
MgS SO2 Which of the following formulas represents a molecular compound A. The first element is given its element name.

Chemistry 101 Naming Binary Molecular Compounds Youtube

Inorganic Nomenclature Ppt Download

The Plan Finish Section A2 2 Naming Ionic Molecular Compounds Ppt Download

Chapter 7 Chemical Formulas And Chemical Compounds Ppt Video Online Download

Binary Compounds List Examples What Is A Binary Compound Video Lesson Transcript Study Com

Naming Writing Formulas For Binary Molecular Compounds Video Lesson Transcript Study Com
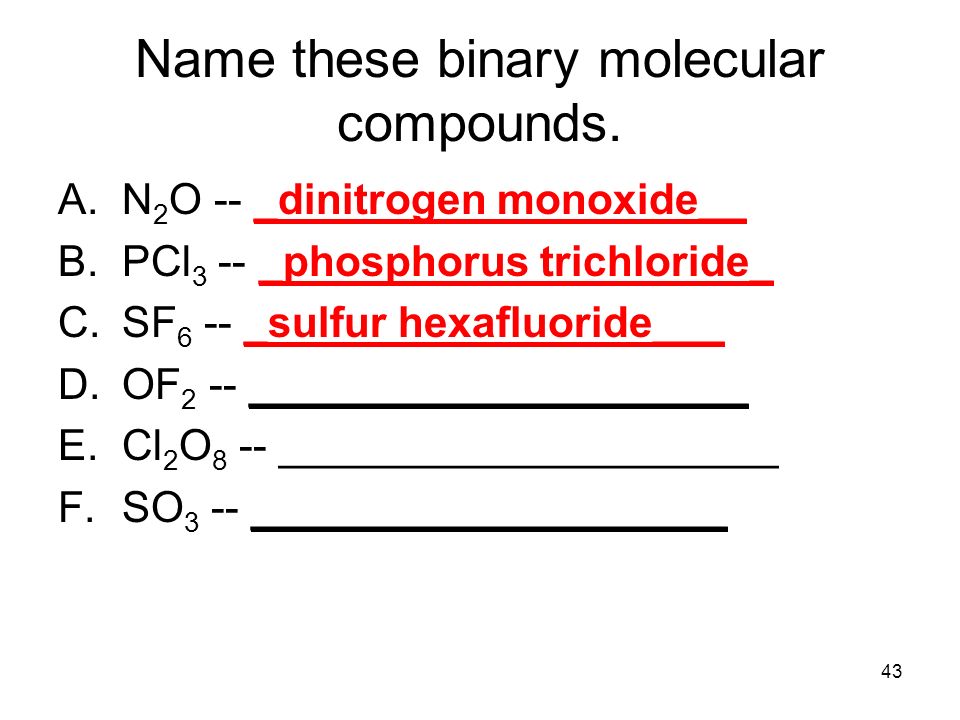
Unit Ii Note Pack Pages Ppt Video Online Download

1 Chapter 3 Chemical Compounds 2 Chemical Formulas Molecular And Ionic Substances The Chemical Formula Of A Substance Is A Notation Using Atomic Symbols Ppt Download
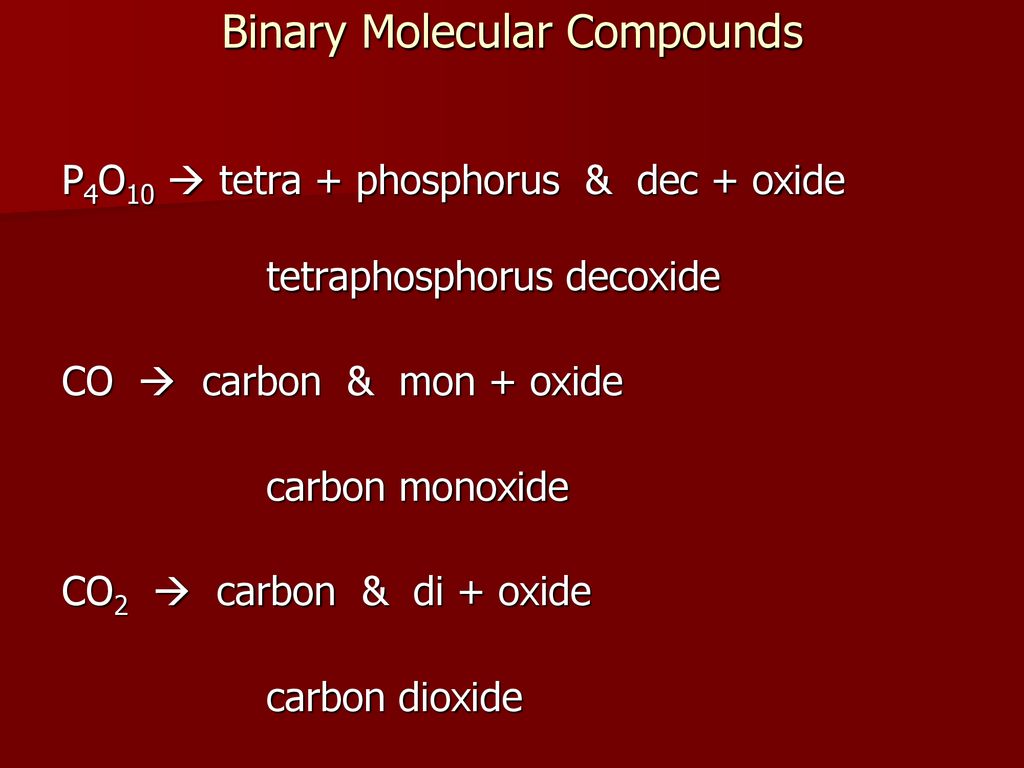
Modern Chemistry Chapter 7 Chemical Formulas Chemical Compounds Ppt Download

Naming Ionic And Molecular Compounds Ppt Download

Chemical Names And Formulas Ppt Download
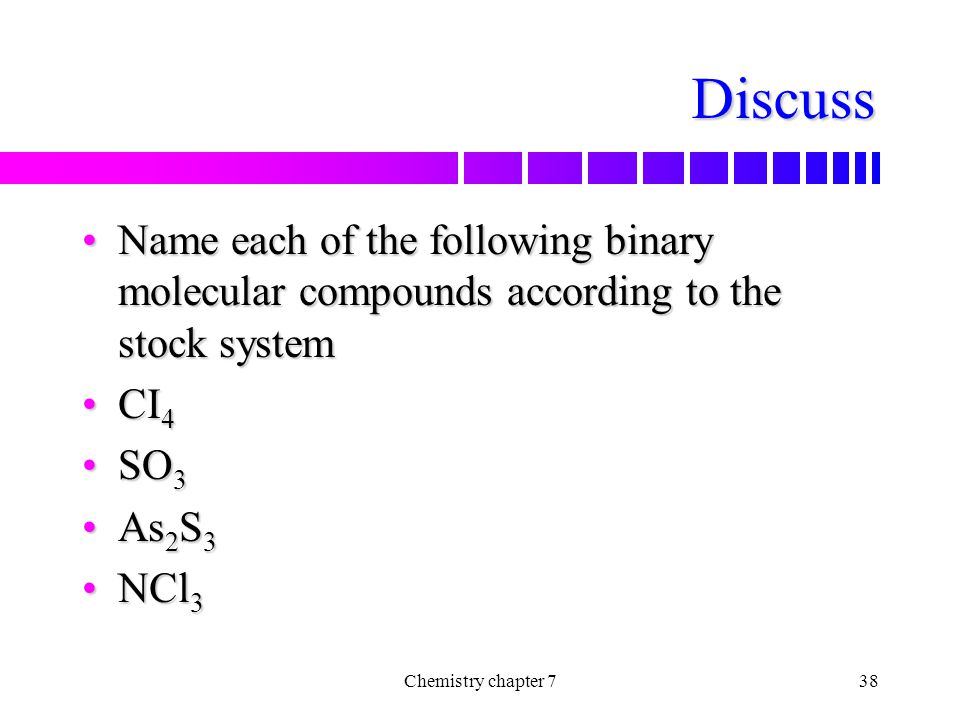
Chemical Formulas And Chemical Compounds Ppt Video Online Download

Molecular Compounds Ionic Compounds Naming Inorganic Compounds All Compounds Can Be Written As A Full Name Or As A Chemical Formula A Formula Provides Ppt Download

Introduction To Chemical Bonding Ppt Download

Nomenclature Ppt Video Online Download

Modern Chemistry Chapter 7 Chemical Formulas Chemical Compounds Ppt Download
Solved What Determines The Order In Which The Component Elements Of Binary Molecular Compounds Are Written


Comments
Post a Comment